Chemistry for Kids
Isotopes
About Atoms and Elements We learned in the atoms and elements sections that each element has its own unique atom which is made up of a specific number of protons. The number of protons determines the atomic number of the element. Each atom also has the same number of electrons as protons.
What is an isotope?
Isotopes are atoms that have the same number of protons and electrons, but a different number of neutrons. Changing the number of neutrons in an atom does not change the element. Atoms of elements with different numbers of neutrons are called "isotopes" of that element.
Naming Isotopes
Since neutrons have no electrical charge, changing the number of neutrons does not affect the chemistry of the element. It does, however, change the mass of the element. Isotopes are identified by their mass, which is the total number of protons and neutrons.
There are two ways that isotopes are generally written. They both use the mass of the atom where mass = (number of protons) + (number of neutrons). The first way is to put the mass as a superscript before the symbol of the element:
- 4He
- 14C
- 235U
- helium-4
- carbon-14
- uranium-238
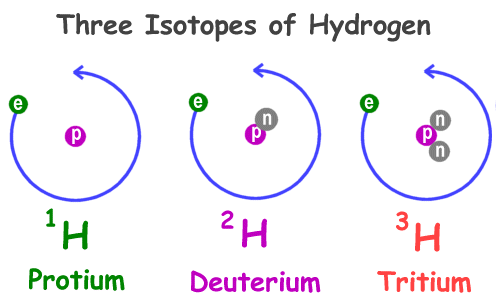
Hydrogen is the only element where the isotopes are given specific names. Common hydrogen, which has zero neutrons, is called protium. Hydrogen with one neutron is called deuterium and hydrogen with two neutrons is called tritium. See the picture at the top of the page.
How many isotopes can an element have?
All elements have a number of isotopes. Hydrogen has the fewest number of isotopes with only three. The elements with the most isotopes are cesium and xenon with 36 known isotopes.
Stable and Unstable Isotopes
Some isotopes are stable and some are unstable. When an isotope is unstable it will decay over time and eventually it will turn into another isotope or element. Unstable isotopes are considered radioactive. Most elements that are found in nature are made up of stable isotopes. The element with the most stable isotopes is tin which has ten different stable isotopes.
Interesting Facts about Isotopes
- Many elements only exist in an unstable or radioactive form.
- All non-natural or man-made elements are radioactive isotopes.
- Heavier isotopes tend to react more slowly than lighter isotopes of the same element.
- Deuterium (the hydrogen isotope with one neutron) can form water with oxygen. This is called "heavy water" as deuterium has twice the mass of normal hydrogen (protium).
- There are 254 known stable isotopes and 80 elements which have at least one stable isotope.
- Twenty-six elements only have one stable isotope. These elements are called monoisotopic.
Комментариев нет:
Отправить комментарий