Radioactivity

Stable and Unstable Isotopes
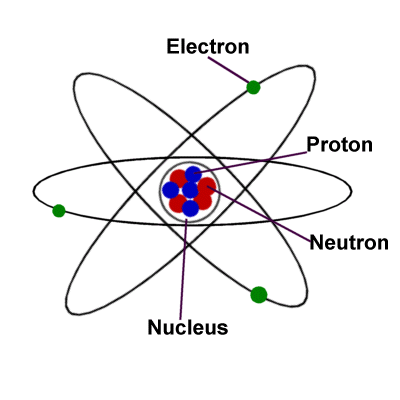
Elements can be made up of different isotopes. Isotopes are atoms with the same number of protons and electrons, but a different number of neutrons. Sometimes isotopes are stable and happy. These are the elements that we see around us and find in nature. However, some isotopes are unstable. These isotopes are called radioactive isotopes. You can go here to learn more about isotopes.
What is radioactive decay?
When isotopes are unstable they emit energy in the form of radiation. There are three main types of radiation or radioactive decay depending on the isotope.
Different Types of Radioactivity
- Alpha decay - Alpha decay is caused when there are too many protons in a nucleus. In this case the element will emit radiation in the form of positively charged particles called alpha particles.
- Beta decay - Beta decay is caused when there are too many neutrons in a nucleus. In this case the element will emit radiation in the form of negatively charged particles called beta particles.
- Gamma decay - Gamma decay occurs when there is too much energy in the nucleus. In this case gamma particles with no overall charge are emitted from the element.
Radioactivity is measured using a unit called the "curie". It is abbreviated as "Ci". The curie measures how many atoms spontaneously decay each second. The curie was named after Marie and Pierre Curie who discovered the element radium.
What is the half-life of an isotope?
The half-life of an isotope is the time on average that it takes for half of the atoms in a sample to decay.
For example, the half-life of carbon-14 is 5730 years. This means that if you have a sample of carbon-14 with 1,000 atoms, 500 of these atoms are expected to decay over the course of 5730 years. Some of the atoms may decay right away, while others will not decay for many thousands more years.
The thing to remember about half-life is that it is a probability. In the example above, 500 atoms are "expected" to decay. This is not a guarantee for one specific sample. It is just what will happen on average over the course of billions and billions of atoms.
Radioactive Decay to other Elements
When isotopes decay they can lose some of their atomic particles (i.e. electrons and protons) and turn from one element into another. Sometimes isotopes decay from one unstable isotope into another unstable isotope. This can happen continuously in a long radioactive chain.
An example of a radioactive chain is uranium-238. As it decays, it transforms through a number of elements including thorium, radium, francium, radon, polonium, and bismuth. It finally ends up as a stable isotope as the element lead.
Why is radiation dangerous?
Radiation can alter the structure of cells in our bodies causing mutations which can produce cancer. The more radiation a person is exposed to, the more dangerous it is.
Is some radiation good?
Despite the risks, there are a number of good ways that science has used radiation. These include X-rays, medicine, carbon dating, energy generation, and to kill germs.
Interesting Facts about Radioactivity
- Uranium in the ground can decay into radon gas which can be very dangerous to humans. It is thought to be the second leading cause of lung cancer.
- The half-life of carbon-14 is used in carbon dating to determine the age of fossils.
- Bismuth is the heaviest element with at least one stable isotope. All elements heavier than bismuth are radioactive.
- Radioactivity was discovered by the scientist A. H. Becquerel in 1896.