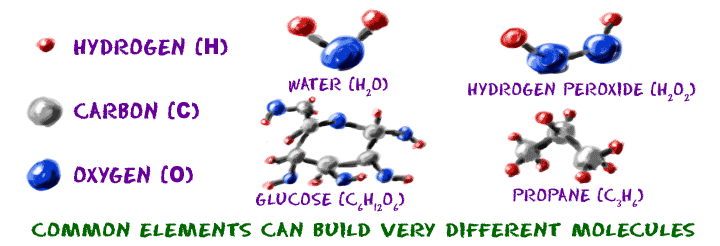
Now we're getting to the heart and soul of the way the Universe works. You know that a generic atom has some protons and neutrons in the nucleus and some electrons zipping around in orbitals. When those pieces start combining in specific numbers, you can build atoms with recognizable traits. If you have eight protons, neutrons and electrons, you will have an oxygen (O) atom. If you have seven protons, neutrons, and electrons, you will have a nitrogen(N) atom. The atoms for each element are unique, even though they are all made of similar subatomic parts.
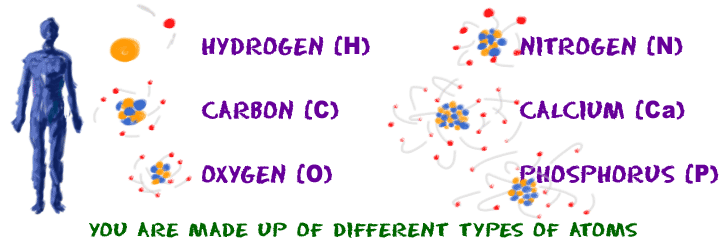
Remember that 'atom' is the general term. Everything is made of atoms. The term 'element' is used to describe atoms with specific characteristics. There are almost 120 known elements. For example, you are made up of billions of billions of atoms but you probably won't find more than 40 elements (types of atoms) in your body. Chemists have learned that over 95% of your body is made up of hydrogen (H), carbon (C), nitrogen, oxygen, phosphorus (P), and calcium (Ca).
With the tools you learn here, you can explore and understand the Universe. You will never stop discovering new reactions and compounds, but the elements will be the same.
(1) Electrons fit nicely into three orbitals. Remember that the orbitals are the places you will generally find the electrons as they spin around the nucleus.
(2) These eighteen elements make up most of the matter in the Universe.
(3) It's a lot easier to remember facts about 18 elements than over 100 elements.
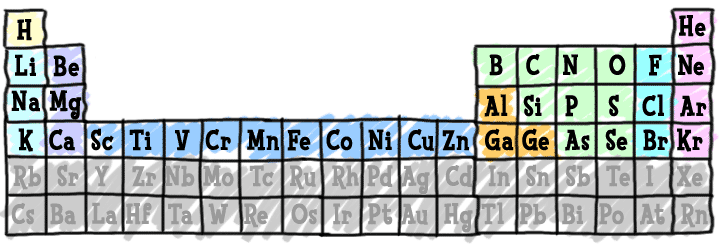
As we move past the first eighteen elements, you can start to learn about transition elements in the fourth period (row) of the periodic table. The transition metals have electron configurations that are a little different from the first eighteen. Make sure you understand the basics of electron orbitals before you move on to this row.
Remember that 'atom' is the general term. Everything is made of atoms. The term 'element' is used to describe atoms with specific characteristics. There are almost 120 known elements. For example, you are made up of billions of billions of atoms but you probably won't find more than 40 elements (types of atoms) in your body. Chemists have learned that over 95% of your body is made up of hydrogen (H), carbon (C), nitrogen, oxygen, phosphorus (P), and calcium (Ca).
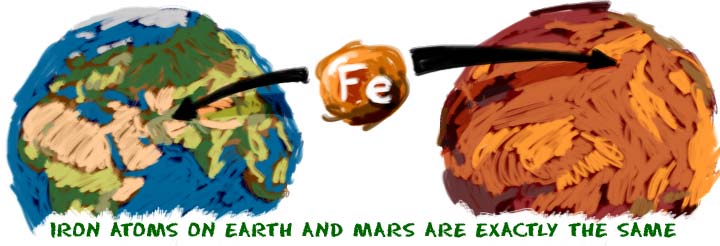
As far as we know, there are a limited number of basic elements. Up to this point in time, we have discovered or created about 120. Scientists just confirmed the creation of element 117 in 2014. While there are more elements to discover, the basic elements remain the same. Iron (Fe) atoms found on Earth are identical to iron atoms found on meteorites. The iron atoms in the red soil of Mars are also the same.
With the tools you learn here, you can explore and understand the Universe. You will never stop discovering new reactions and compounds, but the elements will be the same.
The List of Elements
Since the launch of the site, we've been asked, "Why start with 18?" The rules for the first eighteen elements are very straightforward:
(1) Electrons fit nicely into three orbitals. Remember that the orbitals are the places you will generally find the electrons as they spin around the nucleus.
(2) These eighteen elements make up most of the matter in the Universe.
(3) It's a lot easier to remember facts about 18 elements than over 100 elements.
| Element 1: Hydrogen Element 2: Helium Element 3: Lithium Element 4: Beryllium Element 5: Boron Element 6: Carbon Element 7: Nitrogen Element 8: Oxygen Element 9: Fluorine | Element 10: Neon Element 11: Sodium Element 12: Magnesium Element 13: Aluminum Element 14: Silicon Element 15: Phosphorus Element 16: Sulfur Element 17: Chlorine Element 18: Argon |
| Element 19: Potassium Element 20: Calcium Element 21: Scandium Element 22: Titanium Element 23: Vanadium Element 24: Chromium Element 25: Manganese Element 26: Iron Element 27: Cobalt | Element 28: Nickel Element 29: Copper Element 30: Zinc Element 31: Gallium Element 32: Germanium Element 33: Arsenic Element 34: Selenium Element 35: Bromine Element 36: Krypton |