Separating Mixtures
Many of the substances we use everyday were actually once part of a mixture. Someone somewhere separated that substance from the mixture so we could use it. It turns out that many compounds and elements aren't found in nature in their pure form, but are found as parts of mixtures. Separating substances from mixtures is an important part of chemistry and modern industry. Some important chemistry terms are used in this section including mixtures, suspensions, andsolutions. You can click on the links to learn more about each of them.
Why do we want to separate mixtures?
All the way back to Ancient History, industrious humans have separated mixtures in order to obtain the specific substances that they need. One example of this is extracting metal from ore in order to make tools and weapons. We'll discuss some other examples of separation below.
Separation Processes
The way in which different substances in a mixture are separated is called a process. There are a number of different processes used for separation. Many of them are very complex and involve dangerous chemicals or high temperatures. A lot of important industries in the world today are based on separation processes.
Filtration
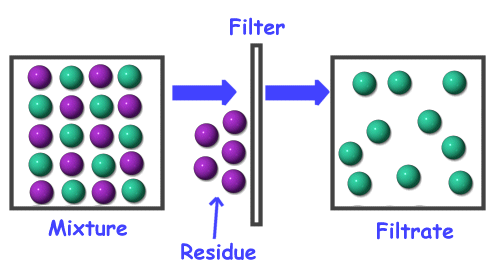
One common method of separation is filtration. Filters are used everywhere. We use them in our houses to filter dust and mites out of the air we breathe. We use them to filter impurities from our water. We even have filters in our bodies such as our kidneys which act as filters to get bad stuff out of our blood.
The filtration process is generally used to separate a suspension mixture where small solid particles are suspended in liquid or air. In the case of filtering water, the water is forced through a paper that is made up of a very fine mesh of fibers. The water that has been run through the filter is called the filtrate. The particles that are removed from the water by the filter are called the residue.
Another common separation process is called distillation. Distillation uses boiling to separate mixtures of liquid solutions. It takes into account that different substances in the mixture will have different boiling points.
For example, if you heat salt water the water in the solution will boil before the salt. The water will then evaporate leaving the salt behind. If the steam from the water is collected it will turn back into liquid as it cools. This cooled water will be pure water without any salt.
| Centrifuge In some cases, there are suspension mixtures where the solid particles are too fine to be separated with a filter. In these cases, sometimes a centrifuge is used. Centrifuges are mechanical devices that spin at very high speeds. These high speeds allow the solid particles in suspensions to settle very quickly. For example, rather than wait for sand to slowly settle to the bottom of water, a centrifuge can cause the sand to settle in a matter of seconds. Some examples of how centrifuges are used include separating blood into plasma and red cells, separating cream from milk, and separating uranium isotopes for nuclear power plants. | 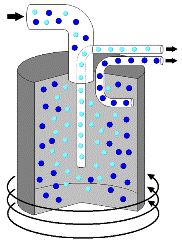 The heavier particles move to the outside of the cylinder as the centrifuge spins allowing the mixture to be separated. |
Other Processes
There are many other separation processes such as sublimation, adsorption, crystallization, and chromatography. Sometimes it takes many stages of processes to get to the final result. One example of this is the processing of crude oil. Crude oil uses many levels of fractional distillation to produce a number of different products including gasoline, jet fuel, propane gas, and heating oil.
Interesting Facts about Separating Mixtures
- To separate liquid solutions where the substances have similar boiling points, a more complex version of distillation is used called fractional distillation.
- Painting uses the separation process of evaporation. The wet paint is a mixture of color pigment and a solvent. When the solvent dries and evaporates, only the color pigment is left.
- The separation process of winnowing was used in ancient cultures to separate the grain from the chaff. They would throw the mixture into the air and the wind would blow away the lighter chaff, leaving the heavier grain.
- High speed centrifuges can spin up to 30,000 times a minute.
- Many separation processes are occurring constantly in nature.
